LIPOATROPHY AND COSMETIC DERMATOLOGY
INJECTABLE FILLER TREATMENTS
Understanding Lipoatrophy
Lipoatrophy leads to a loss of fat tissue under the skin. It causes facial wasting and sinking in the cheek, eye, and temple areas. Often seen in HIV patients, this condition can affect their self-esteem and quality of life.
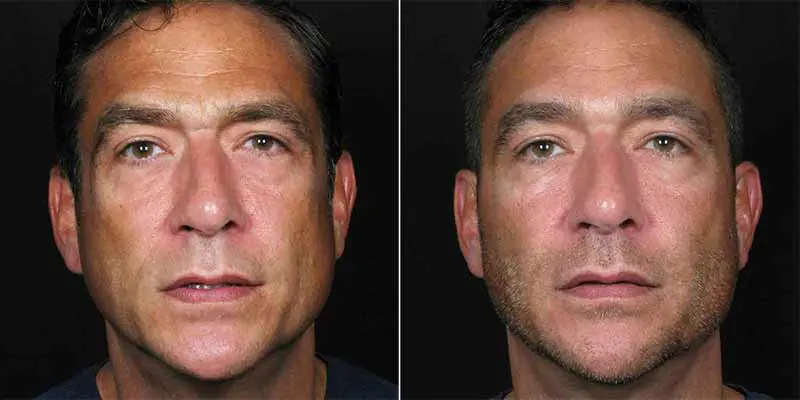
Effective Treatments
Contour Dermatology provides injectable filler treatments to fight lipoatrophy. These treatments aim to restore a more youthful and healthy appearance.
Introducing Sculptra
Sculptra, an FDA-approved injectable filler, is made from poly-L-lactic acid. It is biocompatible and biodegradable. As the first treatment approved for lipoatrophy, insurance may cover the cost if HIV causes the condition.
Dr. Timothy Jochen's Expertise
Dr. Timothy Jochen is a Board Certified Dermatologist. He ranks in the top 1% of facial filler injectors in the nation. He endorses Sculptra as a safe and effective treatment option. Poly-L-lactic acid has been used in dissolvable sutures for over 20 years. There is no need to test for allergic reactions before using Sculptra.
Side Effects
Side effects are generally mild and short-lived. Patients may experience tenderness, redness, and swelling at the injection site. In rare cases, small bumps and larger lumps may appear. Sculptra is not suitable for people with a history of keloid formation or hypertrophic scarring.
Alternative Treatments
Dr. Jochen may recommend Botox or Juvederm as alternatives. Botox temporarily paralyzes facial muscles for a smoother appearance. Juvederm is a hyaluronic acid-based filler that adds volume and smooths wrinkles.
In Conclusion
Contour Dermatology offers various injectable filler treatments. With the help of professionals like Dr. Jochen, patients with lipoatrophy can improve their appearance and quality of life.















