Restylane Lyft Gets FDA Approval
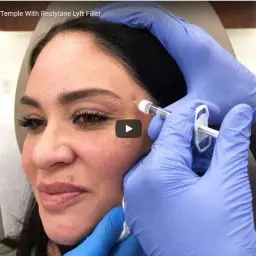
Increase Cheek Volume with this Revolutionary Fillers
There’s a new filler available at Contour Dermatology and we invite you to talk with your provider or schedule a consultation to learn more.
Restylane Lyft FDA Approved for Cheek Volume
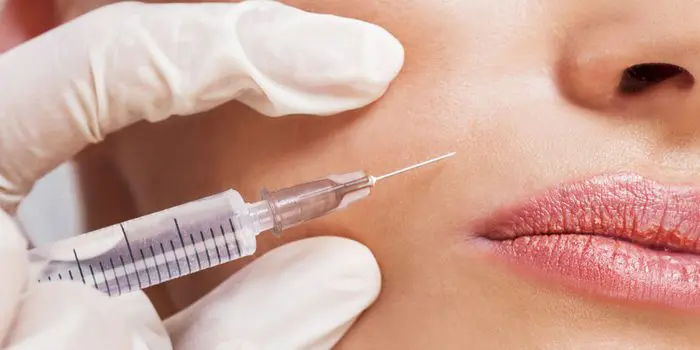
RANCHO MIRAGE, Calif., Sept. 24, 2015 /PRNewswire/ — The FDA recently approved Restylane Lyft, an injectable gel used to increase volume in cheeks and smile lines, making it the first of its kind to be approved for both purposes. Those familiar with the respected Restylane family of injectable fillers will be interested to learn the product is actually Perlane-L. The latter has always been distinguished by its larger particle size and subsequent advantages. Perlane-L will henceforth be known as Restylane Lyft. Galderma, the manufacturer, rebranded the injectable following additional FDA approval to more clearly reflect its dual benefits. Like other members of the Restylane family of products – which include Restylane®, Restylane-L®, Restylane® Silk and Perlane®-L – Restylane Lyft is a clear gel formulation of hyaluronic acid.
What is Restylane Lyft?
Hyaluronic acid is a naturally occurring substance in the body that adds volume and hydration to the skin. As we age, our bodies produce less hyaluronic acid, resulting in wrinkles, folds, and a loss of facial fullness. Restylane Lyft is designed to replenish this lost hyaluronic acid, providing a smoother, more youthful appearance.
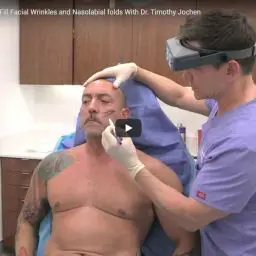
One of the key advantages of Restylane Lyft is its larger particle size compared to other hyaluronic acid fillers. This larger particle size allows for deeper injection into the skin, making it more effective at lifting and volumizing the cheeks. It also makes it suitable for treating deeper wrinkles and folds, such as the nasolabial folds (smile lines).
The rebranding of Perlane-L to Restylane Lyft reflects the manufacturer’s commitment to providing clear and accurate information to consumers and healthcare providers. By aligning the product name with its dual benefits of cheek augmentation and smile line correction, Galderma aims to simplify the decision-making process for those considering this treatment option. The change also allows Restylane Lyft to benefit from the strong reputation and brand recognition that the Restylane family of products has established in the aesthetic medicine market.
Dr. Timothy Jochen's Expertise
Dr. Jochen’s expertise extends beyond his technical skill to his commitment to patient education and personalized care. At Contour Dermatology, he emphasizes the importance of understanding each patient’s unique aesthetic goals and medical needs. This approach ensures that every treatment plan is customized to deliver optimal results while maintaining the highest standards of safety and effectiveness.
In addition to his renowned proficiency with Restylane, Dr. Jochen is actively involved in advancing the field of cosmetic dermatology through ongoing education and research. He regularly attends and contributes to industry conferences and seminars, sharing his insights and learning from other experts to stay at the forefront of cosmetic innovation. This dedication to continuous learning allows him to offer the latest and most effective treatments to his patients.
Contour Dermatology, under Dr. Jochen’s leadership, also prioritizes a holistic approach to beauty and wellness. The practice offers a comprehensive range of services, from advanced skincare treatments to minimally invasive cosmetic procedures. This full-spectrum care model not only addresses immediate aesthetic concerns but also promotes long-term skin health and vitality, ensuring that patients look and feel their best at every stage of life.